ONE treatment that controls heavy menstrual bleeding due
to fibroids1
Study design: ORIAHNN was studied in two 6-month Phase 3 pivotal studies (ELARIS UF-1 AND UF-2) including premenopausal women aged 25-53 with heavy menstrual bleeding associated with uterine fibroids and in an additional 6-month extension study (UF-EXTEND), which did not include a placebo arm1-3
ORIAHNN controls heavy menstrual bleeding due to fibroids
Proportion of women achieving a clinically meaningful bleeding reduction at Final Month1†
ELARIS UF-2
ELARIS UF-1
ORIAHNN
77%
(n=189)
Placebo
11%
(n=94)
P<0.001
Final Month
The last 28 days before and including the last treatment visit date or the last dose date. This can occur anytime from Month 1 to 6 in the pivotal studies.1
Results were similar in ELARIS UF-1 [ORIAHNN (n=206): 69%; placebo (n=102): 9%, P<0.001].
ELARIS UF-1
Results were similar in ELARIS UF-1 [ORIAHNN (n=206): 69%; placebo (n=102): 9%, P<0.001].
What did it take to achieve a
clinically meaningful bleeding
reduction?
≥50% bleeding volume reduction from baseline to Final Month1†
AND
<80 mL bleeding volume
at Final Month1†
=
responder
= responder
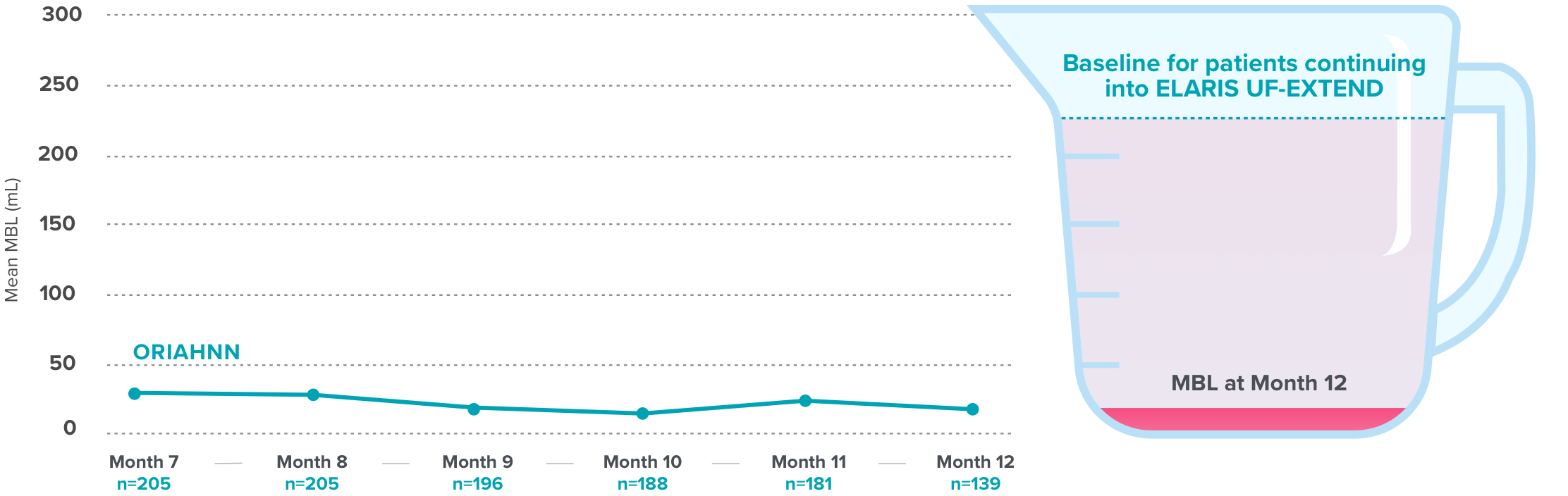
Quick and continued menstrual bleeding reduction with ORIAHNN
Over 50% bleeding reduction at Month 1 and continued reduction seen at Months 3 and 64
Menstrual blood loss (MBL) reduction in ELARIS UF-24



Results were similar in ELARIS UF-1 [Month 1: ORIAHNN (n=187), 52% (124.3 mL) mean reduction, MBL=106 mL; placebo (n=95), 13% (33.1 mL) mean reduction, MBL=226 mL; Month 3: ORIAHNN (n=172), 82% (186.8 mL) mean reduction, MBL=44 mL; placebo (n=85), 4% (0.1 mL) mean reduction, MBL=263 mL; Month 6: ORIAHNN (n=132), 81% (188.5 mL) mean reduction, MBL=41 mL; placebo (n=71), 3% (8.8 mL) mean reduction, MBL=240 mL].
On average, normal menstrual bleeding in women without fibroids is 30-47 mL5,6
Regardless of her characteristics, ORIAHNN controlled her heavy menstrual bleeding
Proportion of women who met the primary endpoint in ELARIS UF-1 and UF-2 at Final Month1,7-12†
Subgroup analyses of the primary endpoint were explored to assess potential differences in treatment effect across subgroup levels and were not controlled for multiplicity.
Click on a subgroup to view results
Select a subgroup to view results
Disease severity subgroups
Baseline ranges and percentages for race and ethnicity depict the entire study population (N=790),¶ while the subgroup analyses depict the ORIAHNN group alone.
72%
Overall pooled results for primary endpoint in ELARIS UF-1 and UF-2 (n=395)7
ORIAHNN delivered clinically significant# improvement in Hgb levels at Month 61,13
In women with anemia who took ORIAHNN
Proportion of women with baseline Hgb ≤10.5 g/dL who achieved a >2 g/dL increase at Month 6
ELARIS UF-2
ELARIS UF-1
ORIAHNN
50%
(n=48)
Placebo
21%
(n=24)
P=0.02
Month 6
Results were similar in ELARIS UF-1 [ORIAHNN (n=52): 62%, placebo (n=31): 16%, P<0.001].
ELARIS UF-1
Results were similar in ELARIS UF-1 [ORIAHNN (n=52): 62%, placebo (n=31): 16%, P<0.001].
Over 90% of women with baseline Hgb ≤10.5 g/dL took supplemental iron.
During the ELARIS UF-1 and UF-2 trials 2 women (0.5%) had heavy menstrual bleeding and required blood transfusion due to anemia (a serious adverse event).
The baseline Hgb in the anemic ORIAHNN-treated women evaluated in this endpoint was ≤10.5 g/dL1
Amenorrhea with ORIAHNN—defined as zero bleeding or spotting2
Proportion of women who experienced Amenorrhea at Final Month2†
Percentage of women with Amenorrhea was a non-ranked secondary endpoint. These results were not controlled for multiplicity.
ELARIS UF-2
ELARIS UF-1
ORIAHNN
53%
(n=172)
Placebo
5%
(n=85)
Final Month
The last 28 days before and including the last treatment visit date or the last dose date. This can occur anytime from Month 1 to 6 in the pivotal studies.1
Results were similar in ELARIS UF-1 [ORIAHNN (n=183): 48%, placebo (n=91): 4%].
ELARIS UF-1
Results were similar in ELARIS UF-1 [ORIAHNN (n=183): 48%, placebo (n=91): 4%].
In ORIAHNN clinical trials, Amenorrhea was defined as having zero bleeding or spotting during the Final Month,† which was a 28-day interval1,2
Quality-of-life and symptom severity results
Health-related quality-of-life scores in ELARIS UF-22,14-17
Total scores for HRQoL range from 0 to 100, with higher scores indicating a better HRQoL
- ORIAHNN
- Placebo
86: HRQoL score for normal control group (women without fibroids)14,15
Symptom severity scores in ELARIS UF-22,14-17
Scores for symptom severity range from 0 to 100, with higher scores indicating increased severity
- ORIAHNN
- Placebo
23: Symptom severity score for normal control group (women without fibroids)14,15
The UFS-QOL is a questionnaire that measures the impact of UF symptoms, including measures of heavy menstrual bleeding on quality of life across 2 scales: HRQoL and symptom severity.14,15
UFS-QOL data from ELARIS UF-1 and UF-2 was a non-ranked endpoint that was not controlled for multiplicity.2
In a study evaluating the UFS-QOL questionnaire, which includes heavy menstrual bleeding measures, 29 control patients had an average symptom severity score of 23 and an average HRQoL total score of 86.14
UFS-QOL=Uterine Fibroid Symptom and Quality of Life.
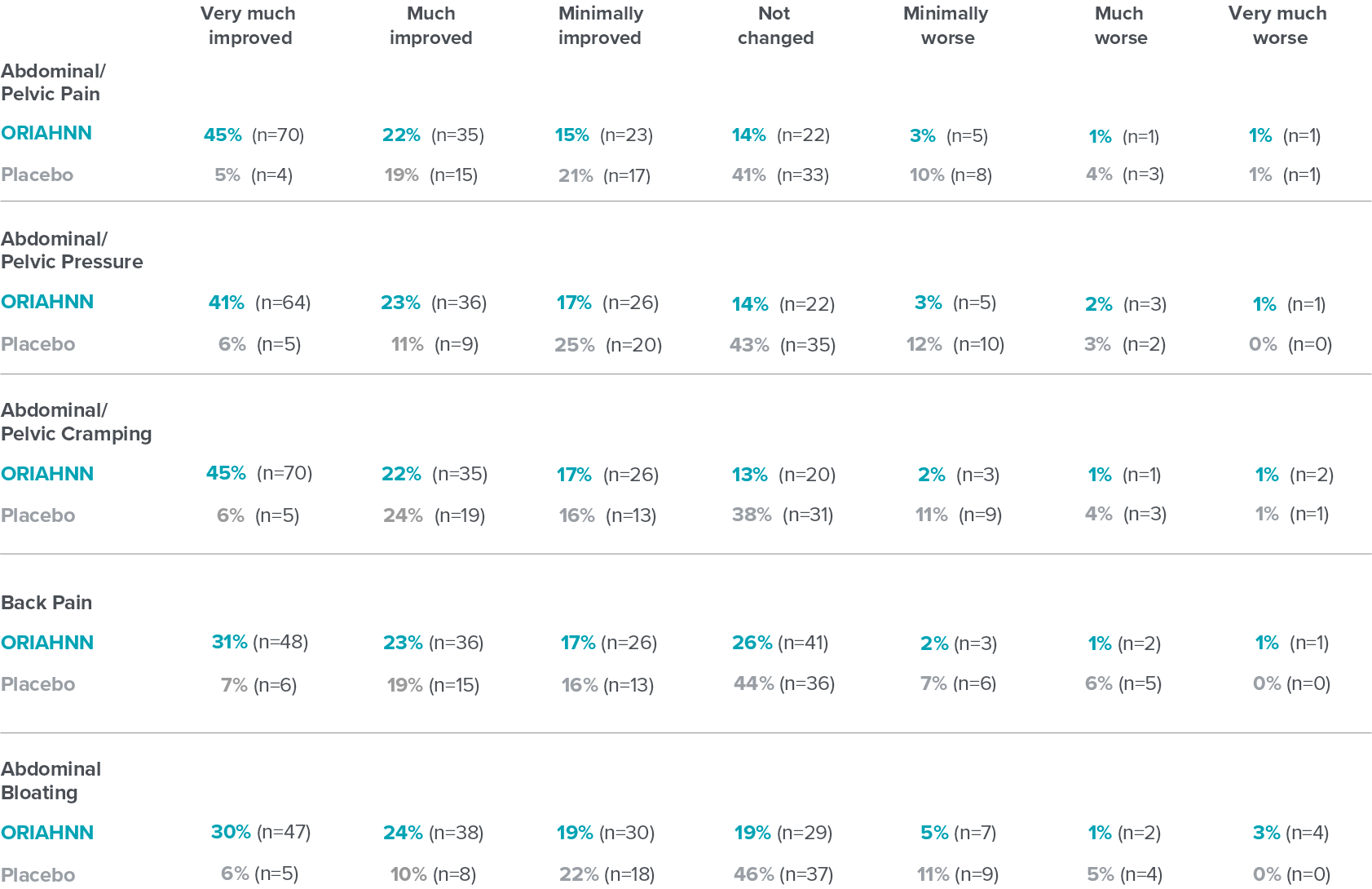
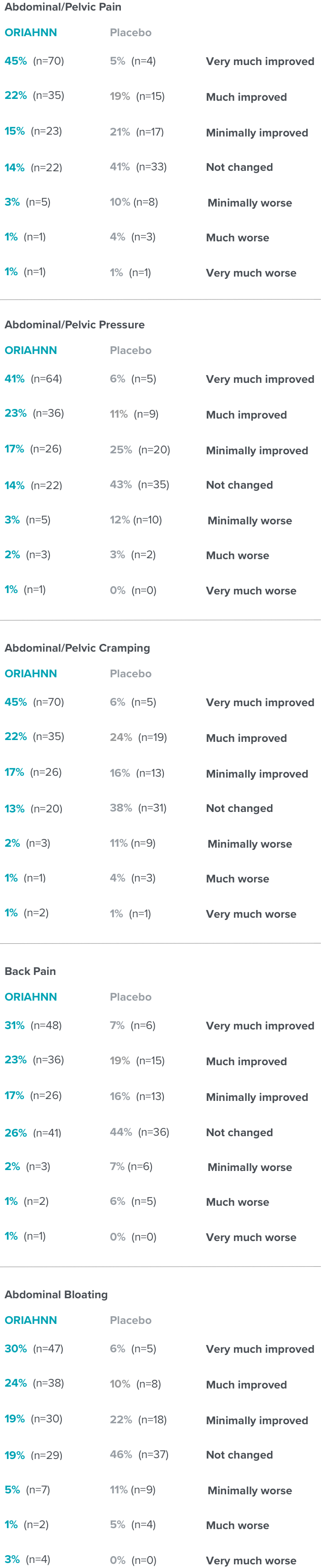
Change in fibroid symptoms
PGIC questionnaire evaluated the assessment of whether severity of symptoms improved or worsened in premenopausal women who suffer from heavy menstrual bleeding due to fibroids18
PGIC results at Month 6 in ELARIS UF-218
Patients' assessment of whether severity of symptoms improved or worsened was a non-ranked endpoint and was not controlled for multiplicity.2
Click on a symptom to view results
Select a symptom to view results
Fibroid symptoms
PGIC=Patient Global Impression of Change.
BMI=body mass index; FIGO score=The International Federation of Gynecology and Obstetrics score for classifying leiomyomas.
†Final Month in ELARIS UF-1 and UF-2 is defined as the last 28 days before and including the last treatment visit date or the last dose date.
‡Submucosal: FIGO score 0-3; Intramural: FIGO score 4; Subserosal: FIGO score 5-8.
§Primary fibroid volume is defined as largest fibroid identified by ultrasound.
||A uterus that is 14-16 weeks in size is approximately 355-460 cm3.
¶For primary fibroid volume only, N=778.
#A >2 g/dL increase in Hgb levels.